COVID-19: A Wake-up Call for How We Treat Wildlife!
Is the coronavirus just the beginning of zoonotic pandemics as habitat and biodiversity loss continue to increase across the globe? Numerous researchers have warned that humanity's destruction of biodiversity creates conditions that allow for new viruses and infectious disease such as Covid-19 to emerge, with severe economic and health impacts for rich and poor countries alike.
The results of habitat and biodiversity loss have implications not only for wildlife, but also human populations around the world, as is evident from the recent and sorrowful pandemic that is unleashing mother natures revenge on the unfortunates who have contracted the coronavirus. In-fact this has even led to a new scientific discipline being created called "planetary health" which focuses on the interconnectedness of the welfare of humans, wildlife and entire ecosystems.
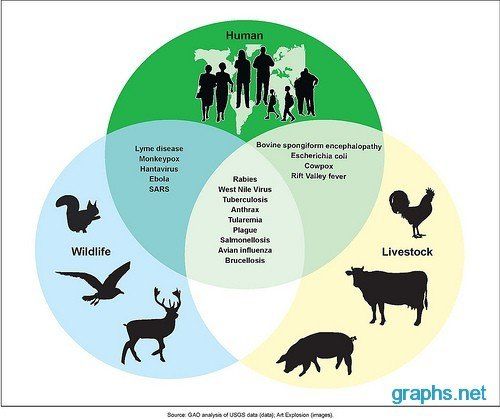
Scientific studies suggest that animal -borne infectious diseases such as Ebola, Sars, bird flu and now COVID-19 (a novel coronavirus) are increasing (Smith et al
., 2014). These zoonotic diseases (also known as zoonoses) are caused by germs, like viruses, bacterial, parasites, and fungi, that spread between animals and people. Scientists estimate that more than 6 out of every 10 known infectious diseases in people can be spread from animals, and 3 out of every 4 new or emerging infectious diseases in people come from animals.
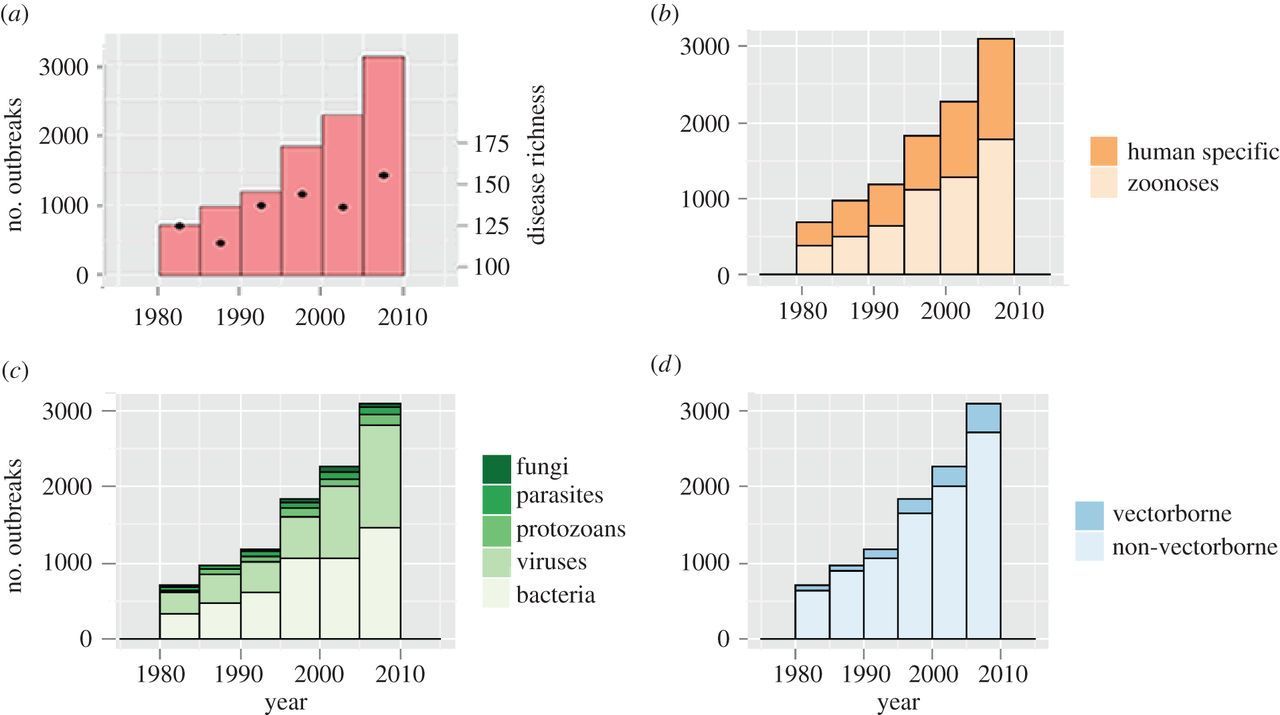
Figure 1. Global number of human infectious disease outbreaks and richness of causal diseases 1980–2010. Outbreak records are plotted with respect to (a) total global outbreaks (left axis, bars) and total number of diseases causing outbreaks in each year (right axis, dots), (b) host type, (c) pathogen taxonomy and (d) transmission mode. (Smith et al., 2014).
According to Kate Jones, chair of ecology and biodiversity at University College London, zoonotic diseases are “ increasing and very significant threat to global health, security and economies ”.
A study by lead author Kate Jones published in the journal Nature
in 2008 indicated that 335 diseases that emerged between 1960 and 2004, at least 60% of which came from animals. Increasingly, these zoonotic diseases are linked to environmental change and human behaviour. The disruption of pristine forests driven by logging, mining, building infrastructure in remote areas
, rapid urbanisation, population growth, overfishing and entering ice free Arctic habitats is bringing people into closer contact with animal species they may otherwise would never have been near before.
Jones described infectious animal-borne diseases as "“ a hidden cost of human economic development. There are just so many more of us, in every environment. We are going into largely undisturbed places and being exposed more and more. We are creating habitats where viruses are transmitted more easily, and then we are surprised that we have new ones
.”
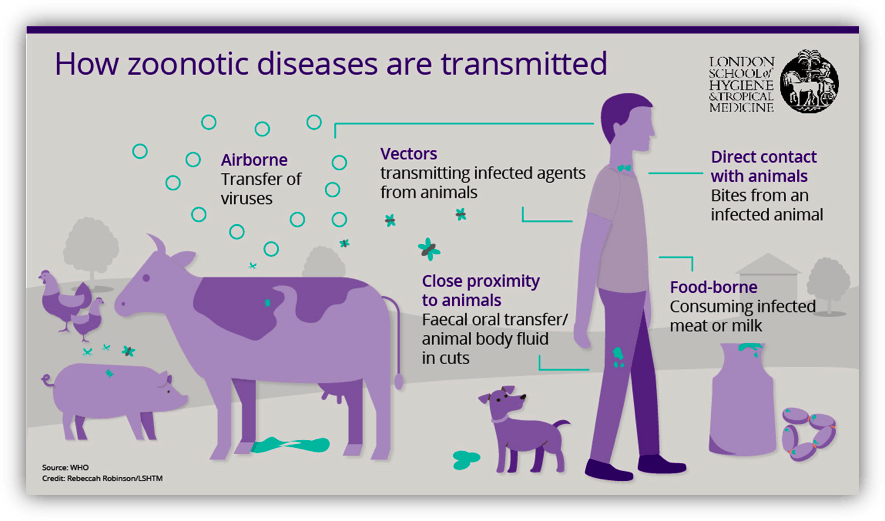
How are zoonotic diseases spread to humans from animals?
- Direct contact: Coming into contact with the saliva, blood, urine, mucous, feces, or other body fluids of an infected animal. Examples include petting or touching animals, and bites or scratches.
- Indirect contact:
Coming into contact with areas where animals live and roam, or objects or surfaces that have been contaminated with germs. Examples include aquarium tank water, pet habitats, chicken coops, barns, plants, and soil, as well as pet food and water dishes.
- Vector-borne: Being bitten by a tick, or an insect like a mosquito or a flea.
- Foodborne: Each year, 1 in 6 Americans get sick from eating contaminated food. Eating or drinking something unsafe, such as unpasteurized (raw) milk, undercooked meat or eggs, or raw fruits and vegetables that are contaminated with feces from an infected animal. Contaminated food can cause illness in people and animals, including pets.
- Waterborne: Drinking or coming in contact with water that has been contaminated with feces from an infected animal.
Scientists have studied seasonal flu for decades. So, despite the danger of it, we know a lot about flu viruses and what to expect each season. In contrast, very little is known about the new coronavirus and the disease it causes, named COVID-19, because it's so new. This means COVID-19 is something of a wild card in terms of how far it will spread and how many deaths it will cause.
What we know about influenza in animals:
Influenza A viruses are found in many different animals, including ducks, chickens, pigs, whales, horses, seals, bats and cats.
Influenza A viruses are divided into subtypes based on two proteins on the surface of the virus: the hemagglutinin (H) and the neuraminidase (N). There are 18 different hemagglutinin subtypes and 11 different neuraminidase subtypes. All known subtypes of influenza A viruses have been found among birds, except subtype H17N10 and H18N11 which have only been found in bats.
Influenza B viruses circulate widely only among humans.
Zoonotic diseases and marine wildlife:
Marine wildlife such as marine mammals may generate a considerable amount of public and scientific interest but also pose a risk to human health from the transmission of zoonotic disease, in addition to traumatic injury. This is a stand-out reason why O.R.C.Ireland does not support "swim with" programmes, although we are often asked if we as scientists get in the water with whales and dolphins, or if we can help member of the public swim with whales in Irish waters. The answer is a" flat-curved" NO.
Most zoonotic marine mammal diseases cause localized skin infections in humans that are treated with appropriate medical therapy. However, other marine mammal zoonoses, if left untreated, induce life-threatening systemic diseases that could pose serious public health risks. Bacterial, viral and fungal marine mammal zoonotic diseases are little known of which also poses a challenge for public health experts, physicians, veterinarians and wildlife biologists.
Contact between marine mammals and humans is increasing as the number of managed animals in aquariums, rehabilitation facilities and research facilities grows. In addition, some countries still hunt marine mammals. Read more here:
Therefore, marine mammal researchers, rehabilitators, trainers, veterinarians, volunteers and hunters (whalers and sealers) have an increased risk of being injured or acquiring zoonotic diseases through extended occupational exposure. As the number of zoonotic diseases rises, more evidence suggests the increase is driven by a complex interplay of environmental (global warming, ocean acidification, pollution), ecological (habitat destruction or fragmentation) and epidemiologic (increasing human densities encroaching on decreasing wildlife populations, global movements of plants and animals) factors (Van Bressem et al
., 2009; Bossart, 2011). Humans are having a major impact on marine environments, with negative impacts on marine mammal populations (Bejder et al
., 2006).
Marine Mammal zoonotic diseases:
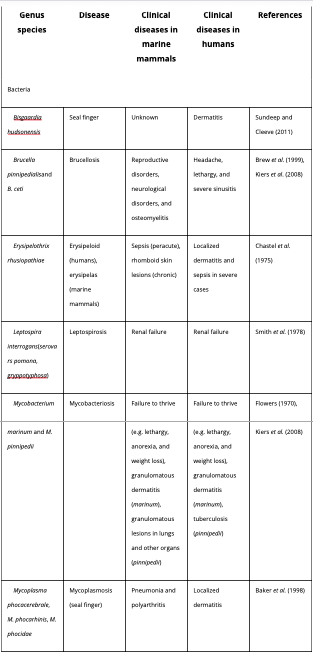
A study investigating the risk of illness transmitted from occupational contact with marine mammals found that more than 10% of the participants reported had contracted localised infections commonly referred to as ‘seal finger’ (Hunt et al ., 2008). ‘Seal finger’ is a result of variety of bacterial and viral species (Table 1). Below we provide a comprehensive review of the bacterial, viral and fungal marine mammal zoonotic diseases, with a synopsis of each disease, its clinical and pathologic manifestation in marine mammals.
Table 1: Bacterial marine mammal zoonotic diseases. (Waltzek et al. , 2012)
Table 2: Viral and fungal marine mammal zoonotic diseases. (Waltzek et al ., 2012)
© Ocean Research & Conservation Ireland (ORCireland) and www.orcireland.ie , est. 2017. If you like our blogs on the latest news in marine science and would like to support our work, visit www.orcireland.ie to become a member, to volunteer or to make a donation today. This article has been composed based on credible sources.
References:
Bejder, L.
,
A. Samuels
,
H. Whitehead
,
N. Gales
,
J. Mann
,
R. Connor
,
M. Heithaus
,
J. Watson‐Capps
,
C. Flaherty
, and
M. Kruetzen
, (
2006)
:
Decline in relative abundance of bottlenose dolphins exposed to long‐term disturbance.
Conserv. Biol.
20
,
1791
–
1798.
Bossart, G. D.
, (
2011)
:
Marine mammals as sentinel species for oceans and human health.
Vet. Pathol.
48
,
676
–
690.
Jones, K., Patel, N., Levy, M. et al.
Global trends in emerging infectious diseases. Nature
451,
990–993 (2008). https://doi.org/10.1038/nature06536
Smith, K., Goldberg, M., Rosenthal. S., Carlson, L., Chen, J., Chen, C., Ramachandran, S., (2014). Global rise in human infectious disease outbreaks. The Journal of the Royal Society Interface.
Van Bressem, M. F., K. Van Waerebeek, J. C. Reyes, D. Dekegel, and P. P. Pastoret, 1993: Evidence of poxvirus in dusky dolphin ( Lagenorhynchus obscurus
) and burmeister porpoise ( Phocoena spinipinnis
) from coastal Peru. J. Wildl. Dis.
29, 109– 113.
Waltzek, T.B., Cortés‐Hinojosa, G., Wellehan Jr., J.F.X. and Gray, G.C. (2012), Marine Mammal Zoonoses: A Review of Disease Manifestations. Zoonoses and Public Health
, 59: 521-535. doi: 10.1111/j.1863-2378.2012.01492.x
SHARE THIS ARTICLE